Description
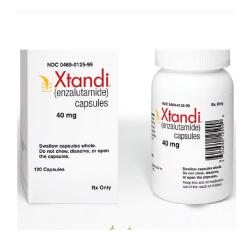
What is the generic name of Xtandi?
Generic name of Xtandi is Enzalutamide
Who is the manufacturer of Xtandi?
Xtandi is manufactured by Astellas Pharma.
Is Xtandi approved by USA FDA?
Yes, Xtandi is approved by FDA. The product was approved in 2012.
What is the dosage of Xtandi?
Xtandi is available as 40mg capsule. 4 capsules (160mg) administered orally once daily. Can be taken with or without food.
What are the storage conditions of Xtandi
The product should be Stored at 20° to 25°C (68° to 77°F). Excursions are permitted between 15°C to 30°C (59°F to 86°F)
How Xtandi is supplied?
Xtandi is supplied as 40mg caspsules.112 capsules packing.
How does Xtandi work?
Enzalutamide acts as a selective silent antagonist of the androgen receptor (AR), the biological target of androgens like testosterone and dihydrotestosterone (DHT). Unlike the first-generation NSAA bicalutamide, enzalutamide does not promote translocation of AR to the cell nucleus and in addition prevents binding of AR to deoxyribonucleic acid (DNA) and AR to coactivator proteins. As such, it has been described as an AR signaling inhibitor in addition to antagonist.The drug is described as a “second-generation” NSAA because it has greatly increased efficacy as an antiandrogen relative to so-called “first-generation” NSAAs like flutamide and bicalutamide. The drug has only 2- to 3-fold lower affinity for the AR relative to DHT, the endogenous ligand of the AR in the prostate gland
What is Xtandi used for?
Xtandi is indicated for the treatment of patients with castration resistant prostate cancer.
Is there any generic or bio similar available for Xtandi.
Xtandi is an innovator brand of Enzalutamide. There are other generic option available for Enzalutamide from reputed manufacturers.
What is the price of Xtandi in India?
You can call or WhatsApp +91-8130290915 or send mail to info@indiangenericmedicines.com to know the price.
Is it safe to buy Xtandi in India from local distributor?
It is always better to check the credential of the person / distributor before confirming. As per the legal guidelines, only patients can import the product after getting proper Documentation.