Description
Imatinib mesylate is a kinase inhibitor (an oral targeted therapy) supplied under the brand name Glivec or Gleevec and approved for the treatment of:
- Newly diagnosed adult and pediatric patients with Philadelphia chromosome-positive (Ph+) chronic myeloid leukemia (CML) in chronic phase (CP).
- Patients with Philadelphia chromosome-positive (Ph+) chronic myeloid leukemia (CML) in blast crisis (BC), accelerated phase (AP), or chronic phase (CP) after failure of interferon-alpha therapy.
- Adult patients with relapsed or refractory (R/R) Philadelphia chromosome-positive (Ph+) acute lymphoblastic leukemia (ALL).
- Pediatric patients with newly diagnosed Philadelphia chromosome-positive (Ph+) acute lymphoblastic leukemia (ALL) in conjunction with chemotherapy.
- Adult patients with myelodysplastic or myeloproliferative diseases (MDS or MPD) linked with PDGFR gene rearrangements.
- Adult patients with an aggressive form of systemic mastocytosis (ASM) without the D816V c-Kit mutation or with c-Kit mutational status unknown.
- Adult individuals with hypereosinophilic syndrome (HES) or chronic eosinophilic leukemia (CEL) who have the FIP1L1-PDGFRα fusion kinase and patients with HES and/or CEL who are FIP1L1 PDGFRα fusion kinase negative or unknown.
- Adult individuals with unresectable, recurrent, or metastatic dermatofibrosarcoma protuberans (DFSP).
- Patients with Kit (CD117) positive unresectable, or metastatic malignant gastrointestinal stromal tumors (GIST).
- Adjuvant treatment of adult patients following resection of Kit (CD117) positive gastrointestinal stromal tumors (GIST).
Dosage and Side Effects of Imatinib
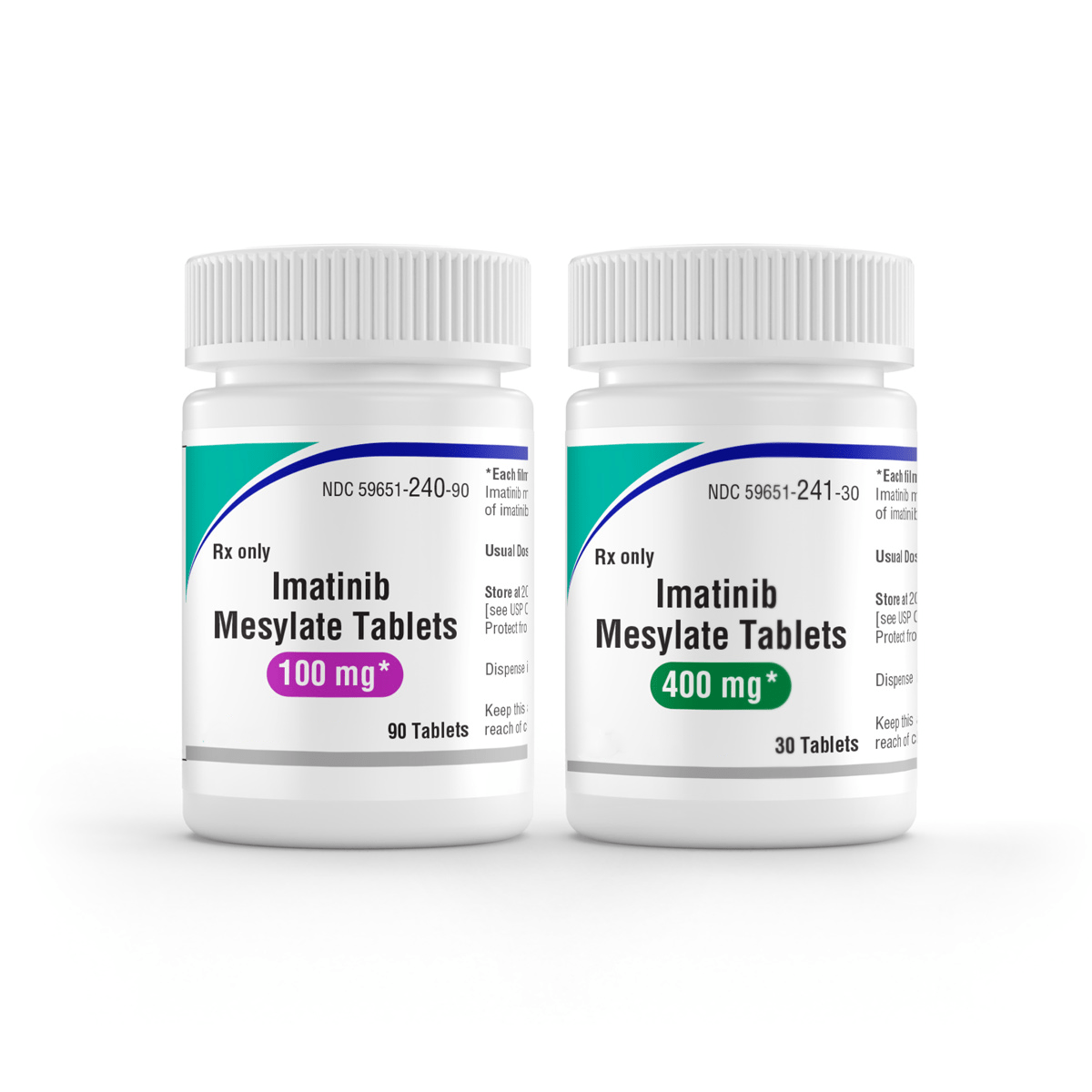
The recommended dosage for adults with Ph+ CML CP is 400 mg/day, for adults with Ph+ CML AP or BC is 600 mg/day, for pediatrics with Ph+ CML CP is 340 mg/m2 /day, for adults with Ph+ ALL is 600 mg/day, for pediatrics with Ph+ ALL is 340 mg/m2 /day, for adults with MDS/MPD is 400 mg/day, for adults with ASM is 100 mg/day or 400 mg/day, for adults with HES/CEL is 100 mg/day or 400 mg/day, for adults with DFSP is 800 mg/day, for adults with metastatic and/or unresectable GIST is 400 mg/day, for adjuvant treatment of adults with GIST is 400 mg/day, for patients with mild to moderate hepatic impairment is 400 mg/day, and for patients with severe hepatic impairment is 300 mg/day.
Administer the prescribed dose orally, with a meal/food and a large glass of water. Administer the doses of 400 mg or 600 mg once daily, whereas administer the 800 mg dose as 400 mg twice daily. For patients not able to swallow the film-coated tablets, the tablets may be dispersed in water or apple juice. Place the required number of tablets in the apt volume of beverage (roughly 50 mL for a 100 mg pill, and 200 mL for a 400 mg pill) and stir well with a spoon. Administer the suspension promptly after the complete disintegration of the tablet(s). For daily dosing of 800 mg and beyond, accomplish the dosing using the 400 mg pill to scale down the exposure to iron. Treatment may be carried on as long as there is no proof of progressive disease or unacceptable toxicity.
The most common Side Effects of Imatinib Tablet are vomiting, edema, nausea, muscle cramps, diarrhea, rash, fatigue, musculoskeletal pain, and abdominal pain.