Why Choose IGM for Your Pharmaceutical Imports?
Global Footprint: Our strong logistics and supply framework spans LATAM, Africa, Europe, Asia, Australia, and the Middle East. This ensures life-saving generic medicines arrive wherever they’re needed—on time and with full compliance.
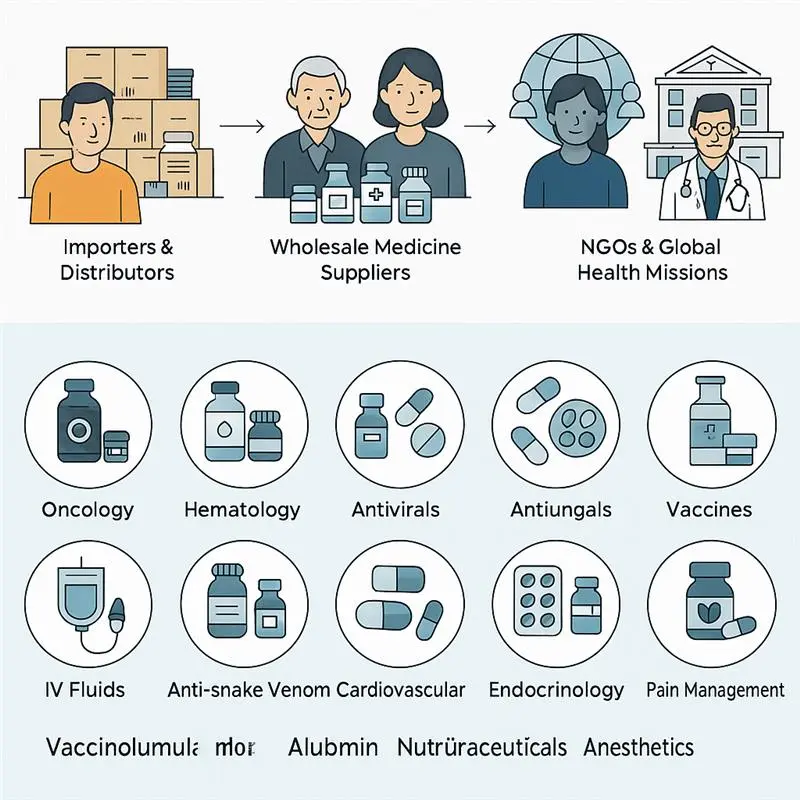
Vast Product Range: We offer access to over 200,000+ branded and generic medicines, covering therapeutic segments such as oncology, nephrology, infectious diseases, and more.
Certified Supply Network: With over 1,000 verified suppliers and 150+ manufacturing partners certified by WHO-GMP, USFDA, EU-GMP, ANVISA, and AMA, we ensure trustworthy sourcing and consistent product quality.
Direct Manufacturer Tie-ups: As a leading pharmaceutical exporter, we work closely with Indian pharmaceutical manufacturers—offering genuine products, clear pricing, and complete traceability across every order.
Versatile Buyer Support: We cater to a broad spectrum of buyers, including global importers, government institutions, NGOs, hospitals, and Named Patient Program (NPP) requests—delivering with precision and regulatory compliance.
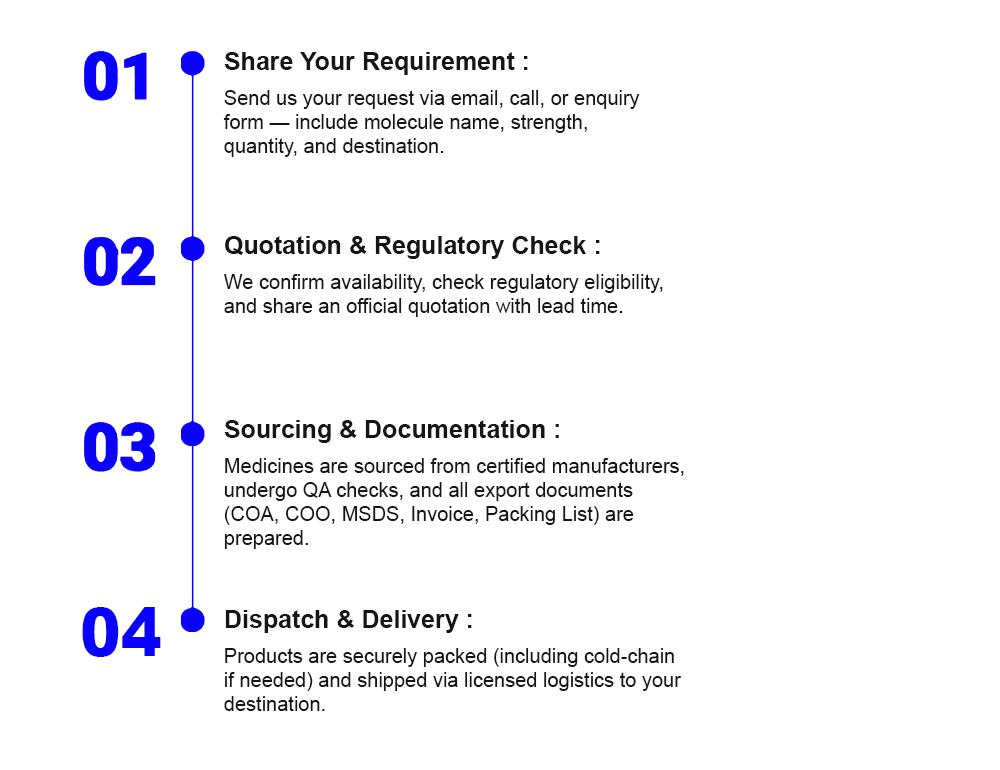
Comprehensive Export Documentation: From Certificates of Analysis (COA) and Origin (COO) to MSDS, commercial invoices, and packing lists, we prepare full documentation aligned with your importing country’s standards.
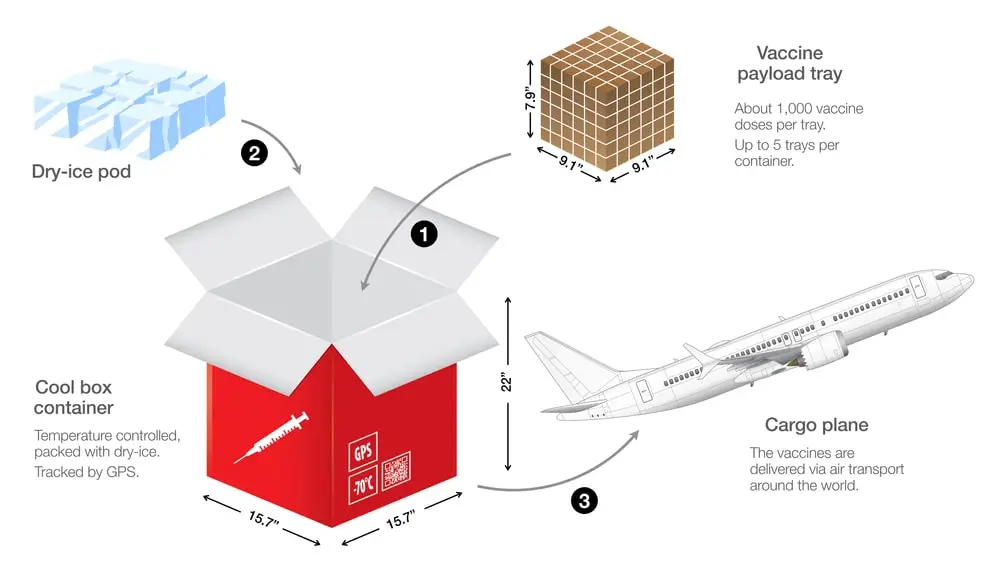
Cold Chain Specialization: We handle sensitive medicines with care—using validated cold-chain packaging and logistics to preserve product integrity during transit.
Regulatory Compliance at Every Step: All exports adhere to international standards—including USFDA, WHO-GMP, ANVISA, SAHPRA, and EMA—ensuring seamless and legal importation.
End-to-End Export Management: From inquiry and sourcing to shipping and post-delivery support, we stand by your side through every stage of the export journey.